December 2022
Control products refer to a substance, material, or article intended by its manufacturer to be used to verify the performance characteristics of an IVD medical device.
Characteristics
| Stability | Stable for 18 months at 2-8°C |
| Interchangeability | Using raw materials and diluent of human-matrix or human-like matrix, the quality control products are interchangeable with human samples. |
| Traceability | According to the requirements of lSO 17511:2003, Getein Biotech established the traceability of its testing system through the reference laboratory, so that the value of the quality control products can be traced to a higher-level reference material or reference method, thereby ensuring the accuracy of the quality control products assignment. |
Specifications
| 1 level x 6 vials x 1 mL | 2 levels x 2 vials x 1 mL | 3 levels x 2 vials x 1 mL |
| 1 level x 3 vials x 1 mL | 2 levels x 1 vial x 1 mL | 3 levels x 1 vial x 1 mL |
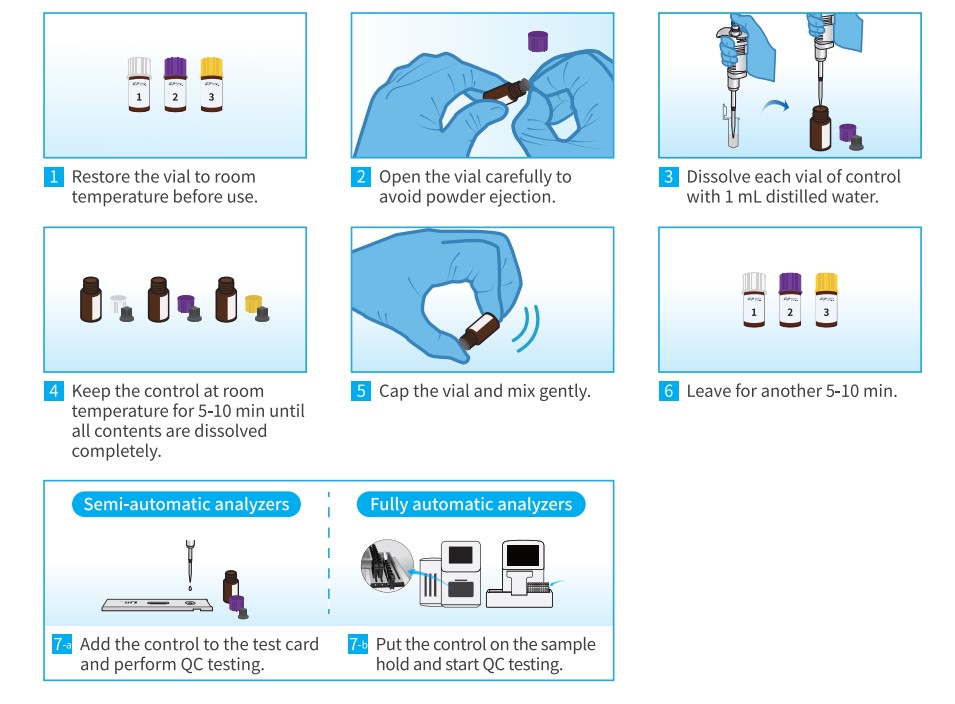
Operation Procedure
Applicable Devices
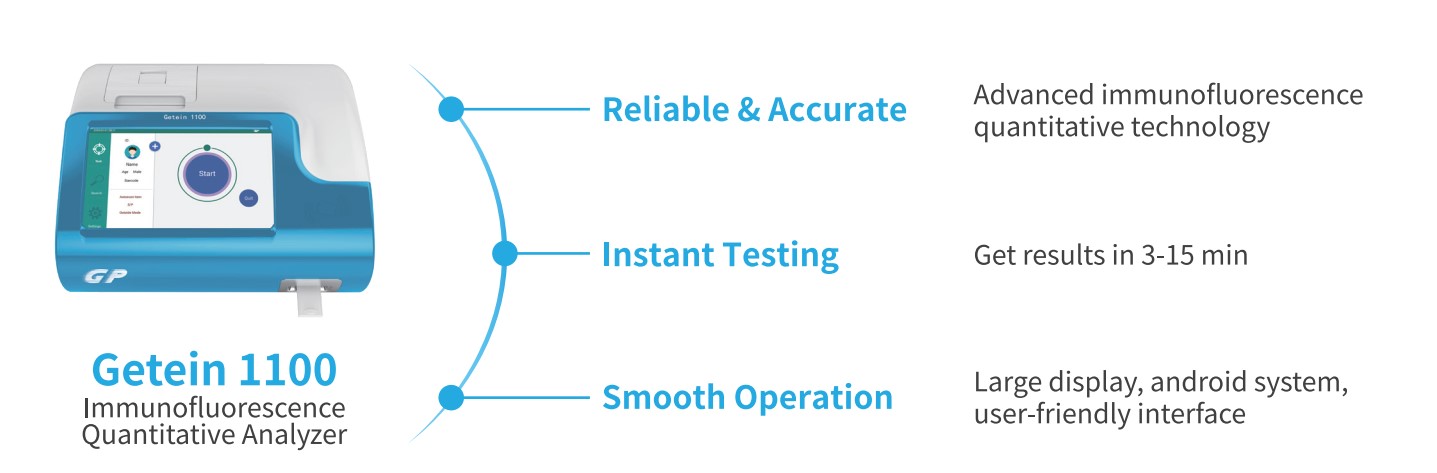

>>Getein 1180<< >>Getein 1160<<

>>Getein 1200<< >>Getein 1600<<
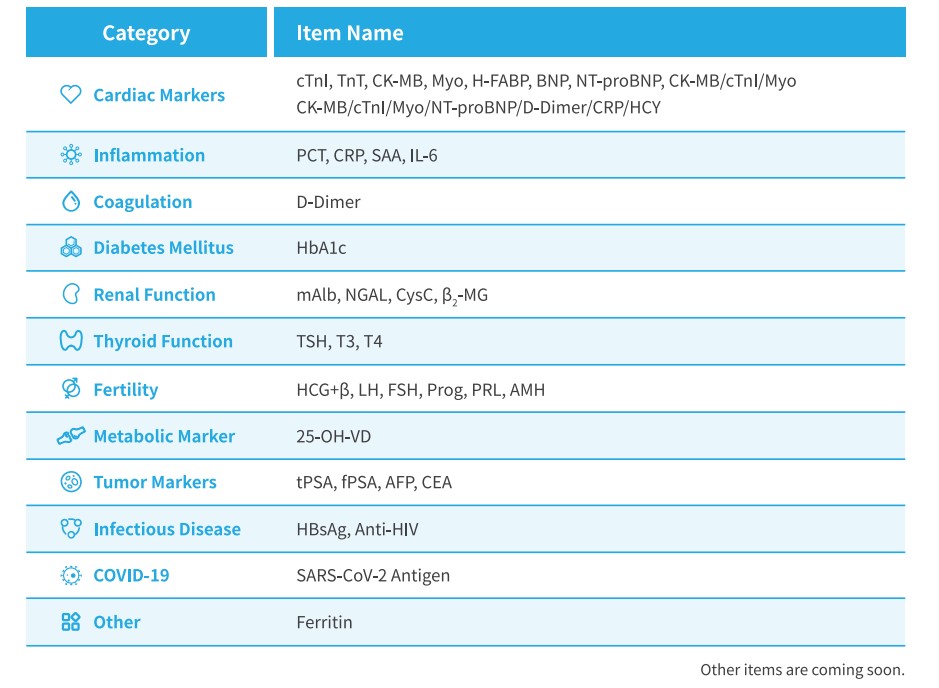
Getein Quality Control Items

Open WeChat and Scan the QR Code. Stay Tuned with Us.