Quality Control Solution
Definition
Substance, material, or article intended by its manufacturer to be used to verify the performance characteristics of an IVD medical device.
Performance Characteristics
One of the parameters used to define the analytical or clinical performance of an IVD medical device.
Basic Features of Control Materials
Internal Quality Control
External Quality Assessment
QC Rules
Specifications for FIA
| 1 level x 6 vials x 1 mL | 2 levels x 2 vials x 1 mL | 3 levels x 2 vials x 1 mL |
| 1 level x 3 vials x 1 mL | 2 levels x 1 vial x 1 mL | 3 levels x 1 vial x 1 mL |
Specifications for Chemiluminescence (CLIA)
| 6 level x 1 vials x 1 mL | 3 levels x 2 vials x 1 mL |
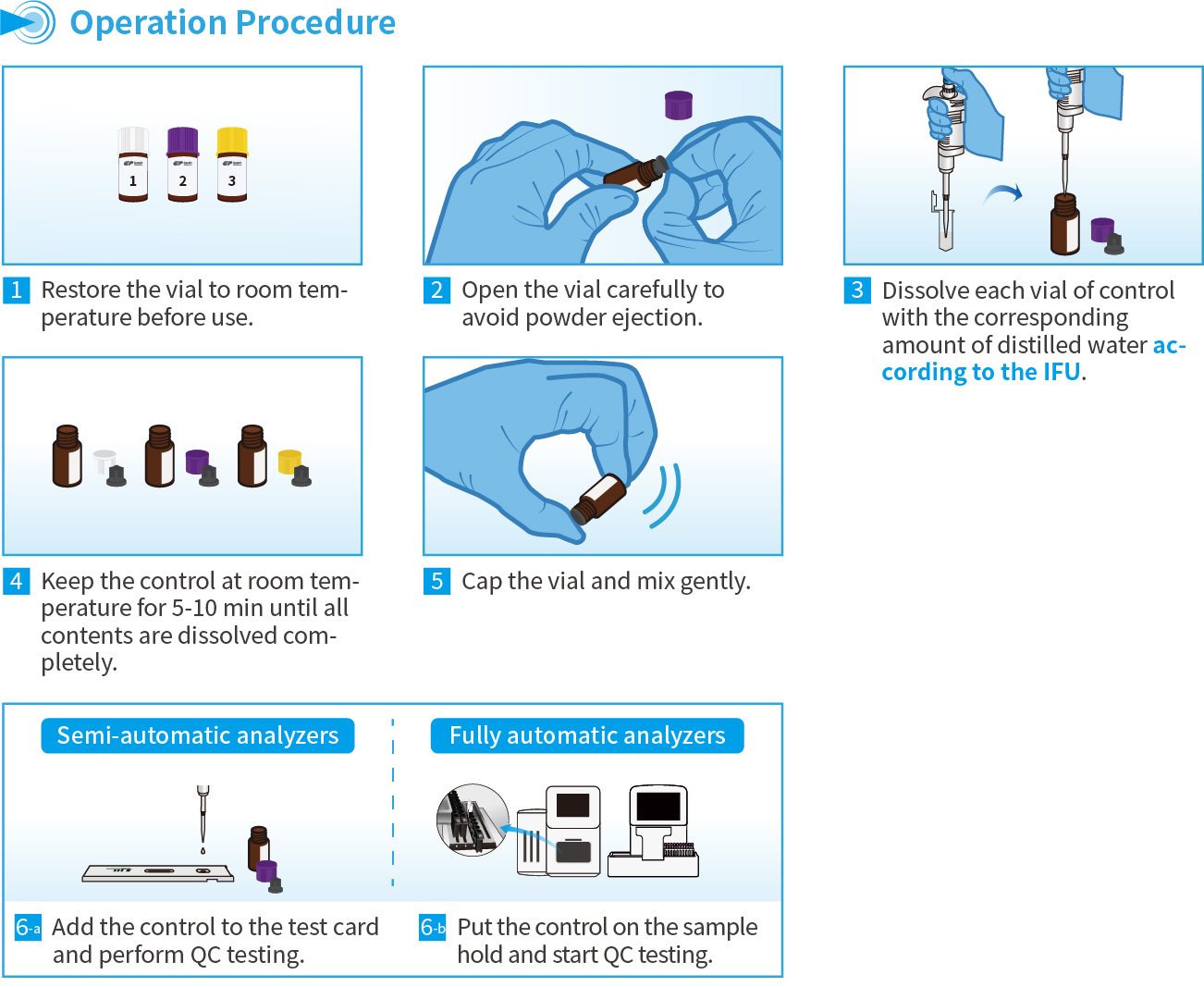
Operation Procedure for FIA
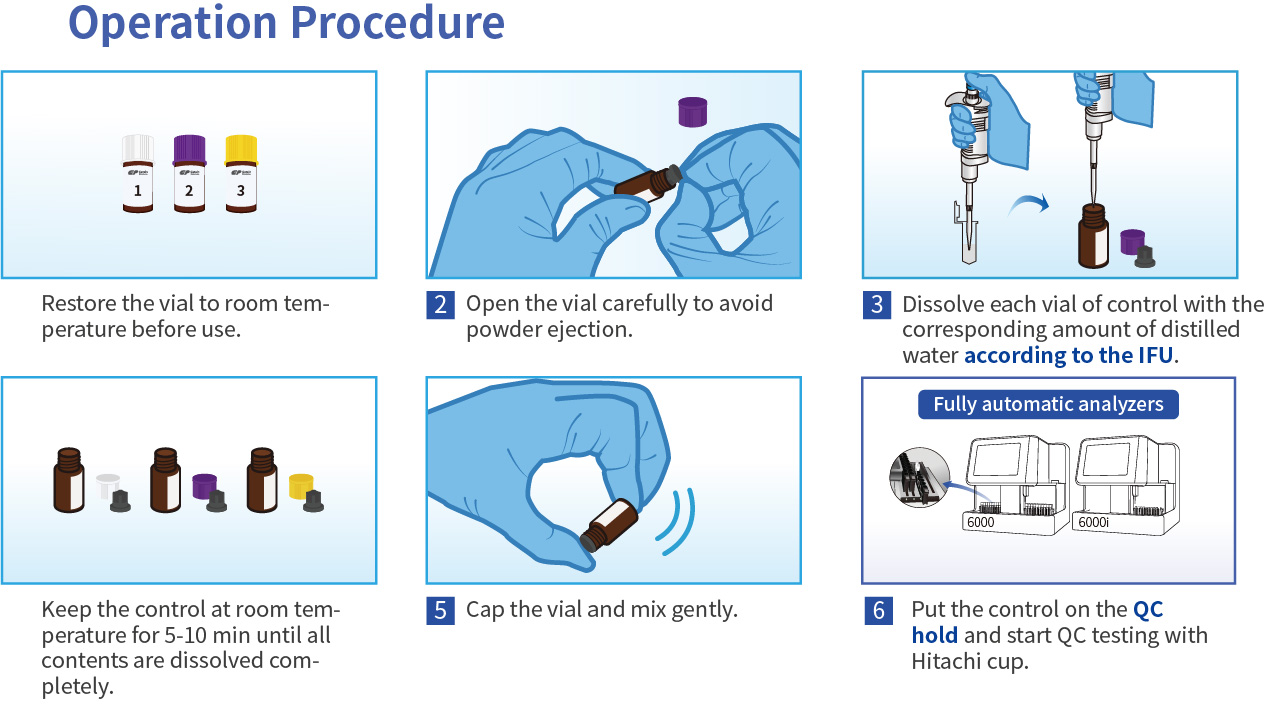
Operation Procedure for Chemiluminescence (CLIA)
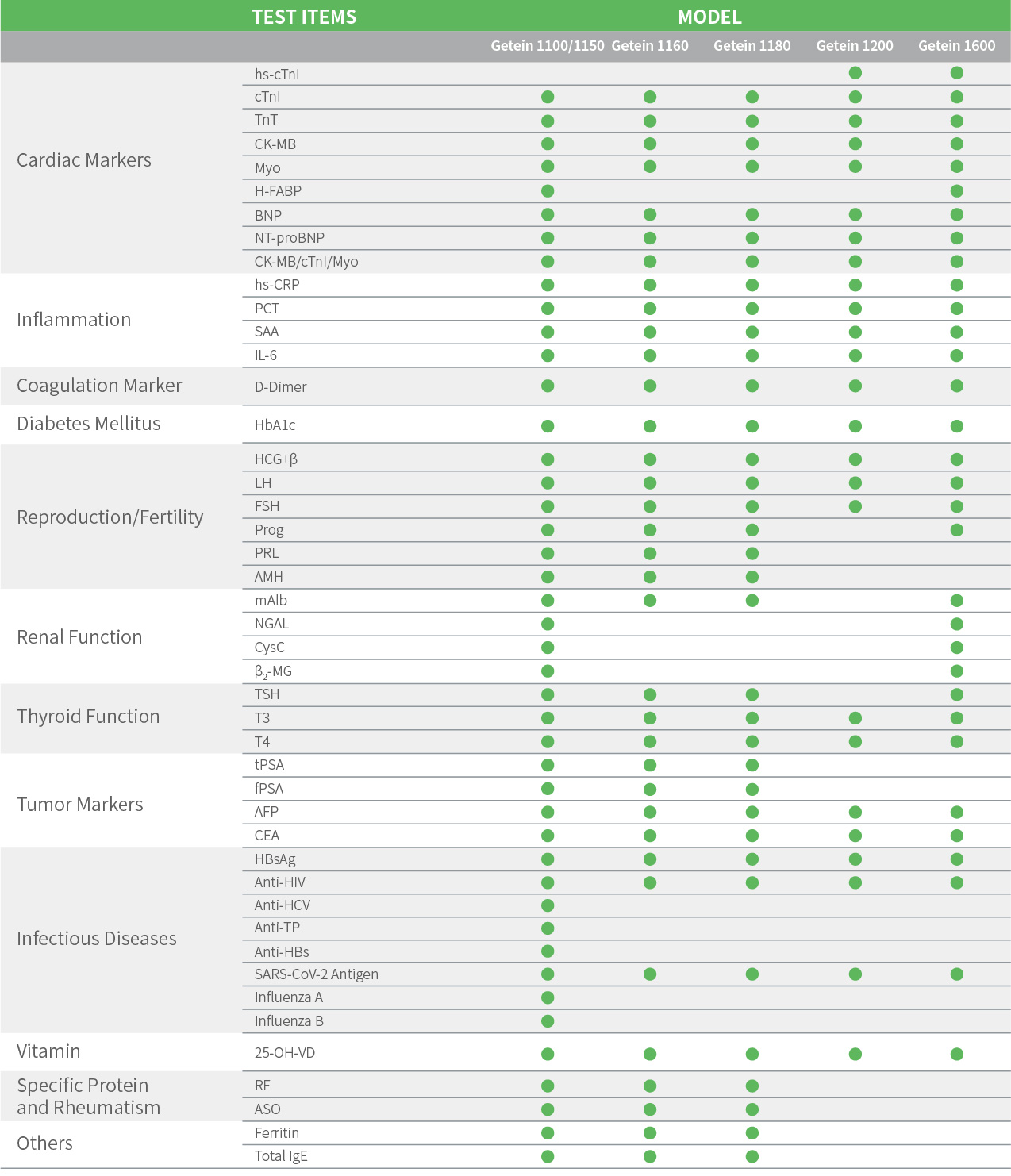
Applicable Devices for FIA
Getein 1150 lmmunofluorescence Quantitative Analyzer
Getein 1100 lmmunofluorescence Quantitative Analyzer
Getein 1160 lmmunofluorescence Quantitative Analyzer
Getein 1180 lmmunofluorescence Quantitative Analyzer
Getein 1200 lmmunofluorescence Quantitative Analyzer
Getein 1600 Immunofluorescence Quantitative Analyzer
Applicable Devices for Chemiluminescence (CLIA)
MAGICL 6000 Chemiluminescence Immunoassay Analyzer
MAGICL 6000i Chemiluminescence Immunoassay Analyzer
MAGICL 6200 Chemiluminescence Immunoassay Analyzer
MAGICL 6800 Chemiluminescence Immunoassay Analyzer
MAGICL 8500 Chemiluminescence Immunoassay Analyzer
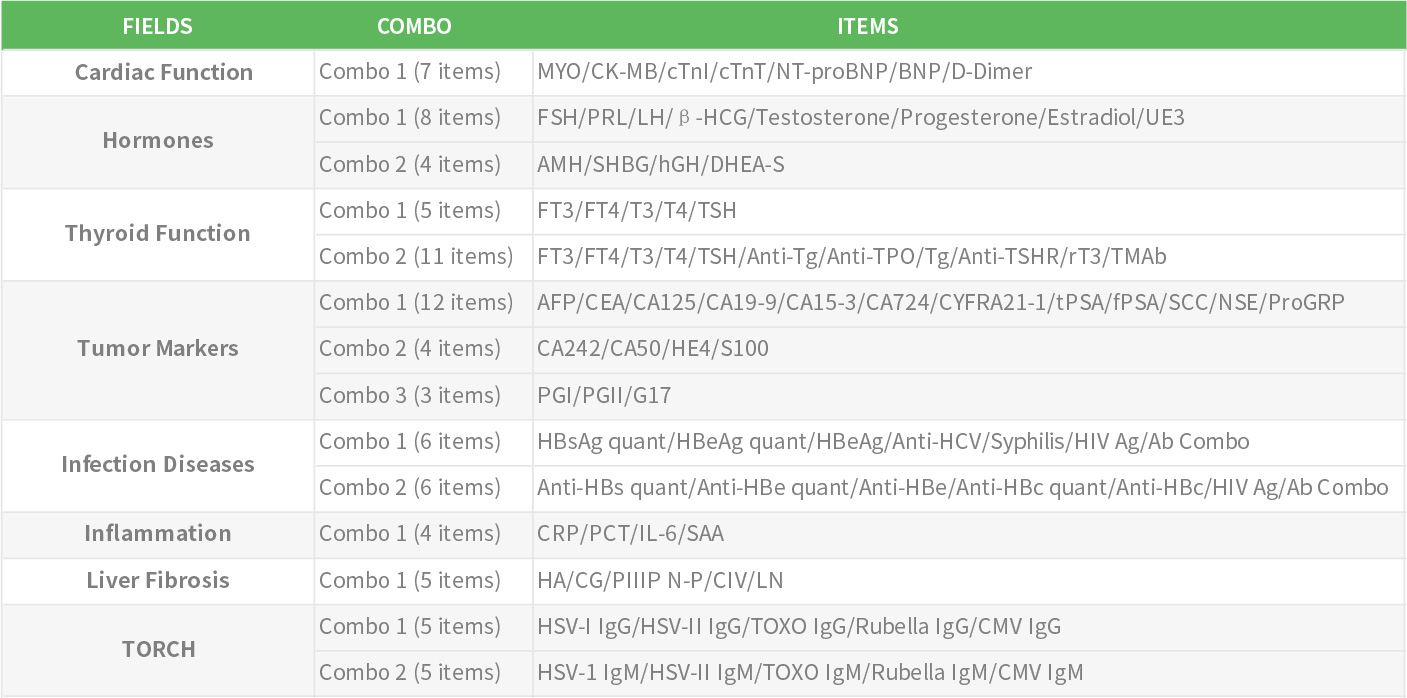
Getein QC Products for FIA
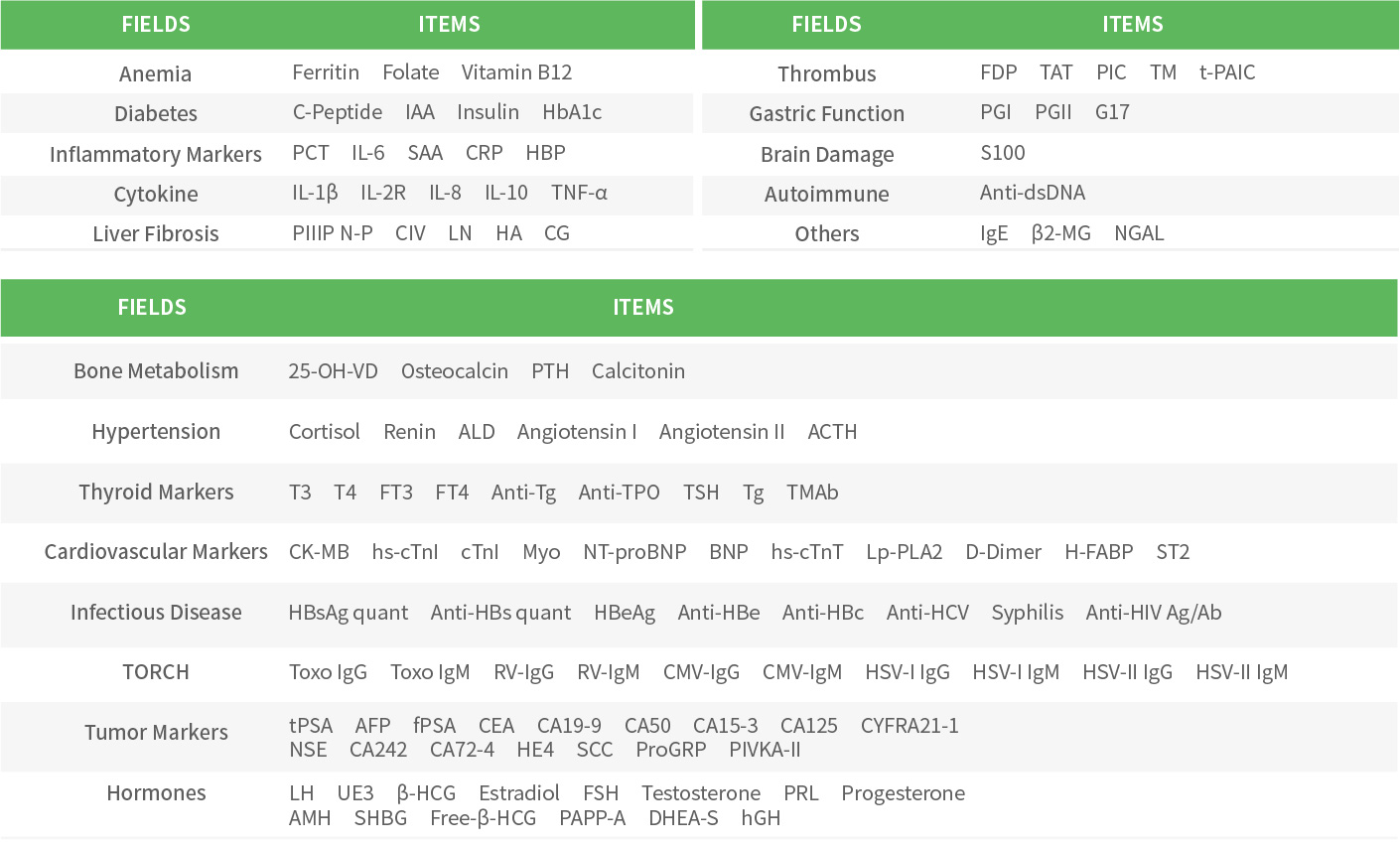
Getein QC Products for Chemiluminescence (CLIA)